Abstract
Introduction: CD22 is widely expressed on leukemic lymphoblasts in patients with B-cell acute lymphoblastic leukemia (ALL). Inotuzumab ozogamicin (InO), an anti-CD22 monoclonal antibody conjugated to calicheamicin, has shown significantly higher remission rates than standard of care (SC) chemotherapy in relapsed or refractory B-cell (R/R) ALL, independently of CD22 expression level. Here we report safety outcomes by CD22 expression in patients with R/R ALL receiving InO (vs SC) as salvage therapy in the INO-VATE trial (NCT01564784).
Methods: Adults with CD22-positive ALL in 1st or 2nd salvage were randomized to InO (n=164; starting dose 1.8 mg/m2/cycle [0.8 mg/m2 on day 1; 0.5 mg/m2 on days 8 and 15 of a 21-28 day cycle for ≤6 cycles]) or SC (n=162; fludarabine/high-dose (HD) cytarabine (Ara-C)/granulocyte colony-stimulating factor, Ara-C plus mitoxantrone, or HD Ara-C).The InO dose was reduced to 1.5 mg/m2 per cycle in patients who achieved complete remission [CR] or CR with incomplete hematologic recover [CRi]. Last patient visit was January 4, 2017. Central flow cytometry was used to assess CD22 expression, which was quantified as % leukemic blasts CD22-positive and as Molecules of Equivalent Soluble Fluorochrome (a quantitative measure of receptor density on leukemic blasts [MESF]). Outcomes were reported in patients by baseline leukemic blast positivity (≥90% vs <90%), and in quartiles of patients (Q1-Q4) defined by CD22 MESF: Q1=lowest CD22 expression, Q4= highest CD22 expression.
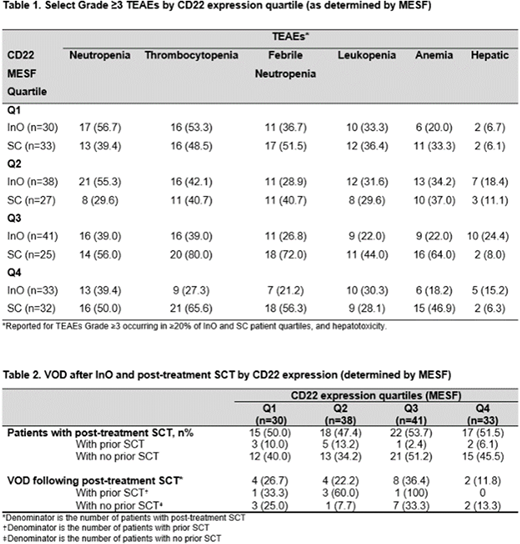
Results: At baseline, 142 InO patients and 117 SC patients had an evaluable sample for central-lab CD22 analysis. The majority of patients in both arms had high (≥90%) CD22 positivity (InO, 75.4%; SC, 72.6%), with a smaller proportion of patients exhibiting CD22 positivity <90% (InO, 24.6%; SC, 27.4%). The median number of completed cycles in the InO arm for the CD22 expression quartiles was n=2 for Q1, n=3 for Q2, n=2 for Q3, and n=3 for Q4; respective median overall dose was similar across quartiles: 4.2 mg/m2 (range: 1.3-9.2), 4.5 mg/m2 (range: 0.8-9.2), 4.2 mg/m2 (range: 0.8-9.3), and 4.6 mg/m2 (range: 0.8-9.6). In both arms, the most common grade ≥3 treatment-emergent adverse events (TEAEs) were neutropenia (InO, 47% vs SC, 44%), thrombocytopenia (InO, 40% vs SC, 58%), febrile neutropenia (InO, 28% vs SC, 55%), leukopenia (InO, 29% vs SC, 34%), and anemia (InO, 24% vs SC, 44%), with similar rates between CD22 MESF expression quartiles (Table 1). Grade ≥3 hepatotoxicity occurred more frequently in the InO arm (17%) vs SC (8%), occurring with similar rates in the CD22 expression quartiles (Table 1). In the InO arm, more patients with ≥90% CD22 positivity received post-treatment SCT (60 [56%] vs 12 [34%] patients with CD22 <90%); 9 (8%) patients with ≥90% CD22 positivity and 2 (6%) patients with <90% also had a pre-treatment SCT. The rate of post-treatment SCT in the CD22 MESF expression quartiles is shown in Table 2. Among patients who proceeded to SCT in the InO arm, where VOD risk was higher, post-transplant VOD occurred in 16 (27%) patients with ≥90% CD22 positivity vs 2 (17%) patients with <90%. When assessing CD22 expression by MESF, the rate of post-transplant VOD was similar across CD22 MESF quartiles (Table 2).
Conclusions: The risk of developing TEAEs, including hepatic adverse events, which were more common in the InO vs SC arm, was not associated with intensity of CD22 expression in either the InO or SC arm. Among InO patients with post-treatment SCT, there was no apparent relationship between CD22 expression and the risk of developing VOD, when analyzing CD22 by % positivity or by MESF.
DeAngelo:BMS: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria; Pfizer Inc: Consultancy, Honoraria; Shire: Honoraria; Takeda: Honoraria; Blueprint Medicines: Honoraria, Research Funding; ARIAD: Consultancy, Research Funding; Amgen: Consultancy; Glycomimetics: Research Funding; Incyte: Consultancy, Honoraria. Advani:Pfizer: Honoraria, Research Funding; Amgen: Research Funding; Novartis: Consultancy; Glycomimetics: Consultancy. O'Brien:Sunesis: Consultancy, Research Funding; Acerta: Research Funding; Pfizer: Consultancy, Research Funding; Aptose Biosciences Inc.: Consultancy; GlaxoSmithKline: Consultancy; Amgen: Consultancy; Gilead: Consultancy, Research Funding; Astellas: Consultancy; Alexion: Consultancy; Abbvie: Consultancy; Kite Pharma: Research Funding; Celgene: Consultancy; Janssen: Consultancy; Vaniam Group LLC: Consultancy; Pharmacyclics: Consultancy, Research Funding; Regeneron: Research Funding; TG Therapeutics: Consultancy, Research Funding. Marks:Novartis: Consultancy; Pfizer: Consultancy; Amgen: Consultancy. Kantarjian:Orsenix: Honoraria; Novartis: Research Funding; Immunogen: Honoraria; BMS: Honoraria, Research Funding; Astex: Research Funding; ARIAD: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Actinium: Honoraria; AbbVie: Honoraria; Pfizer: Honoraria, Research Funding. Wang:Pfizer: Employment, Equity Ownership. Vandendries:Pfizer: Employment, Equity Ownership. Laird:Pfizer: Employment, Equity Ownership. Nick:Pfizer: Employment, Equity Ownership. Stelljes:Amgen: Honoraria; MSD: Consultancy; JAZZ: Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal